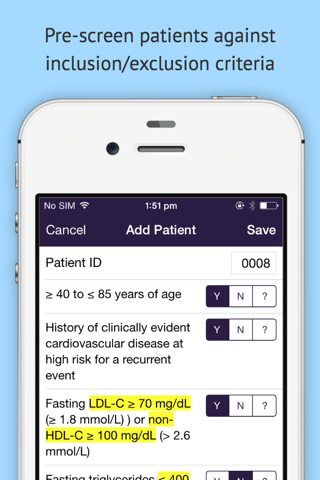
dScreen is the fastest way for sites to pre-screen patients against clinical trial inclusion/exclusion criteria.
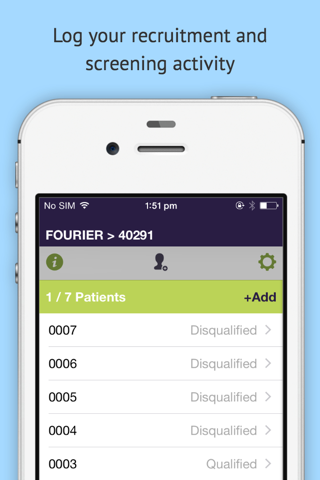
dScreen allows principal investigators and other site staff to rapidly pre-screen patients against clinical trial inclusion/exclusion criteria - either during the chart review process, or at the bedside. When used during the clinical trial planning process, this information provides valuable insight into clinical trial feasibility, patient recruitment and protocol design levers that may affect recruitment. When used during the clinical trial recruitment period, dScreen provides sites with a quick and easy way to screen patients during routine clinic visits without worrying about laminated flowcharts and version control - and provides clinical trial operations with live insight into the screening effort being performed by sites around the world.